Benefits and advantages of Light-Sheet Microscopy for fast, high-resolution tissue imaging
Why light-sheet microscopy is transforming tissue imaging
Light-sheet microscopy is a powerful imaging approach that enables fast, high-resolution volumetric imaging of large tissue samples. In tissue analysis workflows where speed, depth, and preservation of delicate structures matter, light-sheet microscopy is a major advancement over traditional methods such as confocal or widefield imaging.
In this post, we explain what light-sheet microscopy is, why you might choose it, and the key advantages and benefits of using light-sheet microscopy for tissue analysis. We also describe how the 3Di™ Hybrid Open-Top Light-Sheet Microscope is designed to address common challenges in large-scale 3D tissue imaging.
What is Light-Sheet Microscopy
Light-sheet microscopy uses a thin plane of light to illuminate only a single slice of a specimen at a time. This approach enables rapid imaging of whole volumes without exposing the entire sample to high illumination energy, minimizing photobleaching, phototoxicity, and image artifacts.
Unlike point-scanning methods that sequentially expose every voxel, light-sheet microscopy captures an entire plane at once, enabling much faster acquisition. For large tissues or whole organs where true 3D structure and context are critical, light-sheet microscopy offers significant practical advantages
Key benefits of light-sheet microscopy for tissue analysis
Faster 3D imaging with reduced photobleaching
Because light-sheet illuminates only the in-plane region being imaged, it dramatically reduces out-of-focus light exposure. This results in faster acquisition and reduced photobleaching, making it ideal for imaging sensitive fluorophores or large volumes without damage.
High-resolution volumetric imaging over large tissue scales
Light-sheet microscopy is uniquely capable of capturing high-resolution 3D datasets across large tissue volumes. That means you can visualize complex biological structures from the cellular level up to the tissue scale in a single dataset, a major benefit for quantitative spatial biology.
Enhanced tissue context and structural preservation
Because this approach captures entire volumes, it preserves the true spatial context of cells and structures within the tissue. This is especially important for understanding microenvironment interactions or spatial patterns that 2D sections can miss.
Lower phototoxicity for delicate samples
For live or cleared tissue samples sensitive to light exposure, light-sheet illumination reduces cumulative phototoxic stress compared to point-scanning illumination. This makes it easier to maintain signal integrity across deep tissue regions.
What “hybrid open-top light-sheet” means, and why it matters
Traditional light-sheet microscopes place objectives around the sample, which restricts the sample size and complicates mounting. Open-top systems invert this geometry, placing the optics below the specimen. Alpenglow’s hybrid architecture builds on this idea, integrating two coordinated light sheet paths.
• The orthogonal open top path provides fast, wide field scouting across large cleared samples.
• The non-orthogonal dual objective path provides submicrometer zoom with high numerical aperture collection.
The two paths work together. Users can quickly screen large volumes, identify regions of interest, and then re-image specific sites at much higher resolution without changing microscopes or altering sample mounting.
The hybrid concept was introduced and validated in Nature Methods in 2022. The paper describes the non-orthogonal dual-objective (NODO) path, remote refocusing to correct a tilted light sheet, and multi-scale workflows, all of which are demonstrated on intact mouse brains and metastatic lesions.
Before that, the team established the open-top foundation with multi-immersion optics for cleared tissues in Nature Communications in 2019, addressing practical mounting, compatibility with many clearing protocols, and high-throughput imaging. These engineering choices are core to today’s product, the 3Di™.
Hybrid light sheet geometry for wide area scouting and high resolution zoom
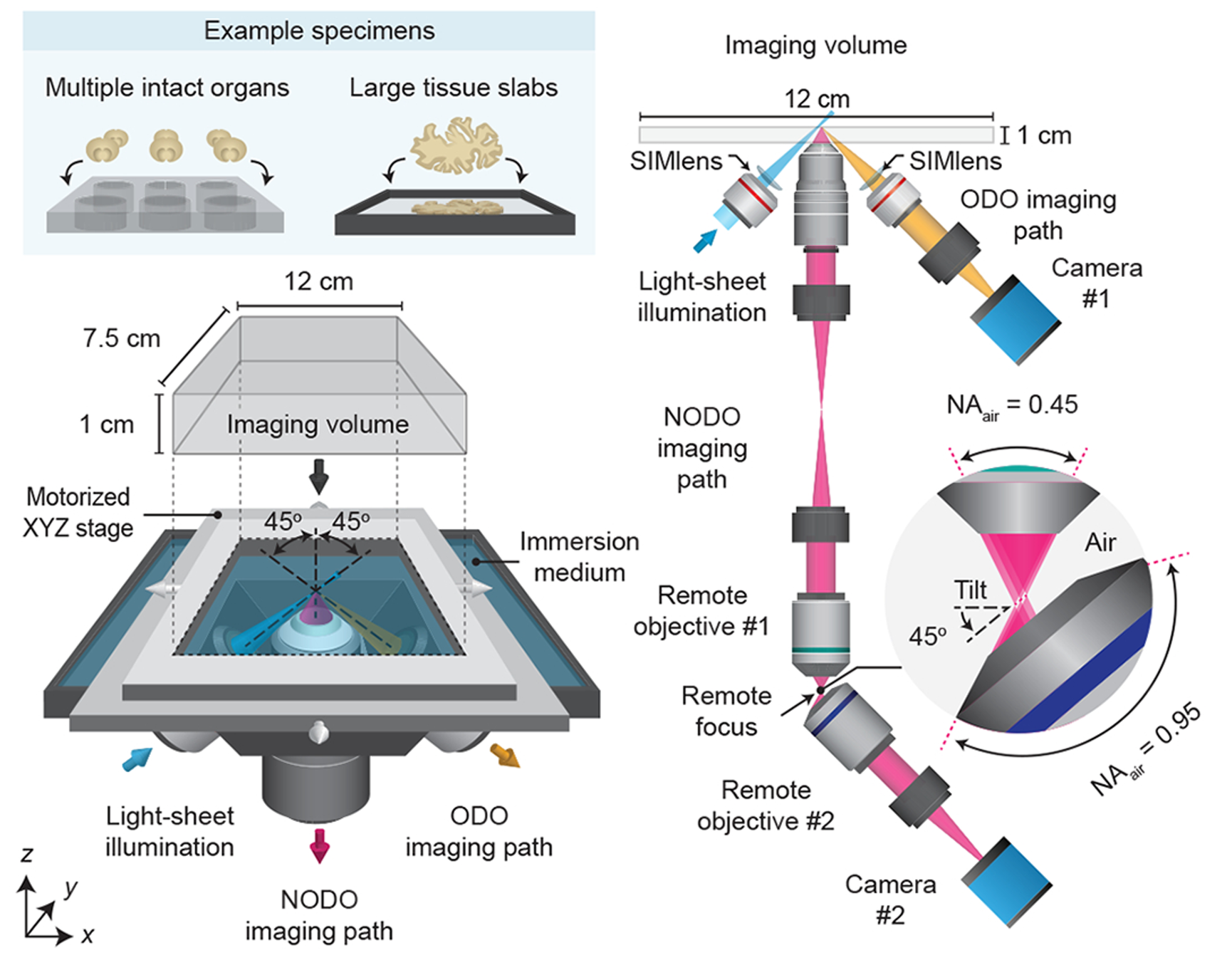
The hybrid microscope architecture consists of three objectives positioned below the specimen. One objective is used for light-sheet illumination, and the other two objectives are used for orthogonal dual-objective (ODO) and non-orthogonal dual-objective (NODO) imaging. By using a motorized stage, tiled imaging is possible with both paths over a large 12 × 7.5 × 1 cm (XYZ) imaging volume, accommodating multiple intact cleared organs and large tissue slabs mounted in an array of specimen holders.
What you can expect from the 3Di™ HOTLS
Two imaging modes on one instrument
Scout (ODO path) for rapid, low-resolution whole-sample surveys and navigation.
Zoom (NODO path) for localized, high-resolution imaging of regions identified during Scout.
Users switch between Scout and Zoom in the same imaging session inside the LUMI interface.
“LUMI is the microscope’s GUI for setup, acquisition, and live monitoring. It supports:
- Intuitive scan setup, so operators configure trays, tiles, and channels quickly.
- Simultaneous live previews in Scout and Zoom, which helps position ROIs and verify focus before committing to long scans.
- A clean, easy interface usable by new and advanced users, with a “see it working” preview”
Large imaging volume with open-top convenience
Tiled imaging over up to ~12 × 7.5 × 1 cm volumes, suitable for multi-sample trays, large organs, or arrays of biopsies.
Open-top holders keep cleared specimens stable by gravity and light compression, while separating immersion and clearing solutions for easier handling.
Resolution and optics at a glance
Zoom path pixel sampling on the order of ~170 nm per pixel, with collection NA up to 0.7 for sub-micrometer detail.
Scout path wide field surveying with pixel sampling around ~2 µm per pixel, used to find and queue ROIs for Zoom.
Multi-immersion optics spanning refractive indices ~1.33 to 1.56 to match common clearing media. 3Di™ HOTLS Alpenglow
This level of sampling corresponds to the practical confocal resolution achieved within a light sheet workflow that maintains speed and minimizes photobleaching.
Throughput and automation
A motorized stage enables raster tiling across large trays, including multi-well plates and multi-specimen formats such as arrays of mouse brains. Multi-sample workflows are fully automated, featuring cloud-based upload, flat-fielding, stitching, registration, and AI-ready outputs.
Comparing Scout and Zoom resolution
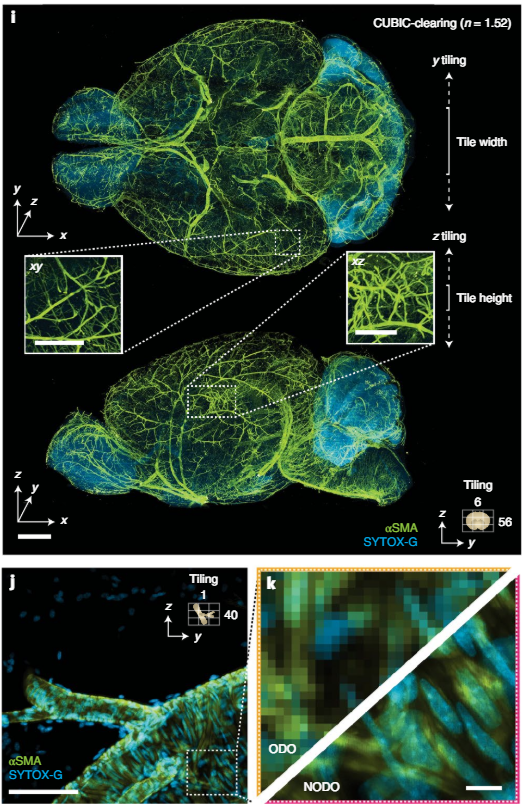
i, Representative ODO imaging results of an entire intact CUBIC-cleared mouse brain with arterial (αSMA) and nuclear (SYTOX-G) staining. The ODO imaging path is able to clearly resolve vasculature in both the xy and xz planes (insets). The size and direction of tiling is annotated. j,k, Targeted NODO imaging of a sub-region centered on a branching arteriole resolves individual smooth muscle cells and sub-nuclear features that are not resolved by ODO imaging. Scale-bar lengths are as follows: i, 1mm (insets, 500 μm); j, 100 μm; and k, 10 μm.
Broader applicability across research, translational, and clinical programs
The 3Di™ system is designed not only for research but also for translational and clinical environments where the analysis of intact human tissue is essential. Applications include human biopsy imaging, 3D pathology for inflammatory skin disease, tumor microenvironment mapping, and non-destructive workflows that preserve precious specimens for sequencing or IHC. This breadth makes the system relevant for academic research labs, core facilities, translational groups, and early-stage clinical programs that require full tissue context and quantitative 3D readouts.
As an example of clinical impact, nondestructive 3D pathology has been used for patient risk stratification in prostate cancer, as demonstrated in the study “Prostate Cancer Risk Stratification via Nondestructive 3D Pathology with Deep Learning Assisted Gland Analysis” (Cancer Research, 2022).
From imaging to answers: 3Di™, 3Dm™, and 3Dai™
Alpenglow Biosciences positions the microscope within an end-to-end platform:
3Di™ for 3D image acquisition,
3Dm™ for data management and correction,
3Dai™ for quantitative analysis, including counts, shapes, distances to vessels, segmentation, and spatial correlations.
This pipeline is designed for non-destructive 3D pathology, preserving tissue for downstream assays and enabling larger, decision-grade volumetric datasets.
Typical samples and clearing compatibility
The instrument was engineered around cleared tissue imaging across protocols with different refractive indices. The open-top layout accepts whole organs, biopsies, organoids, and multi-well plates, with customizable holders to match geometry and workflow. The multi-immersion objective design and open-top mounting were created specifically to accommodate this variety.
Selected example workflows among applications
Whole-organ survey with targeted ROI quantification
Scout the full organ, mark ROIs, then Zoom to quantify axonal density, vascular branching, or tumor invasion margins in situ, as demonstrated in the 2022 Nature Methods paper on intact brain and brain metastasis models. NatureBiopsy-scale 3D pathology with preserved tissue
Image intact biopsies, run 3Dai™ analyses for counts, shapes, and distances, then keep the specimen for sequencing or IHC. The non-destructive approach and data-processing pipeline are described in the 2021 Perspective. NatureHigh-throughput cleared tissue screens
Load multi-sample trays, run automated tiling and cloud correction, and export stitched volumes for downstream AI. This high-throughput use case builds directly on the multi-immersion open-top design reported in 2019. Nature
Key specifications and capabilities
Imaging volume: up to ~12 × 7.5 × 1 cm tiled volume per run.
Dual paths: Scout for speed, Zoom for sub-micrometer detail.
Pixel sampling: ~2 µm per pixel in Scout, ~170 nm per pixel in Zoom.
Collection NA: up to 0.7 on the Zoom path.
Lasers: standard 405 nm, 488 nm, 561 nm, 638 nm. Now up to 5 channels, adding 735 nm or 780 nm
Multi-immersion range: n ≈ 1.33 to 1.56.
End-to-end pipeline: integrated upload, corrections, registration, and quantitative 3D analytics.
For a product overview and screenshots of the LUMI interface, see the HOTLS page: https://www.alpenglowbiosciences.com/3di-hotls-microscope
How it fits into your lab or program
Alpenglow supports different engagement models, from imaging services and pilot projects to placing a full solution on-site with training and support. The FAQ and site overview describe the combined hardware, software, and services approach for research, translational studies, and early clinical investigations.
Where to learn more
Product page: 3Di™ Hybrid Open-Top Light-Sheet Microscope.
Brochure with specs and workflow diagrams, including imaging volume, multi-immersion optics, Scout and Zoom details, and analysis examples. 3Di™ HOTLS Alpenglow
Peer-reviewed papers that underpin the system
Glaser et al., Nature Methods 2022, hybrid HOTLS with NODO and remote refocusing, multi-scale imaging demonstrations. Nature
Glaser et al., Nature Communications 2019, multi-immersion open-top design for cleared tissues and high-throughput workflows. Nature
Liu, Glaser, Madabhushi, Nature Biomedical Engineering 2021, Perspective on non-destructive 3D pathology and its computational stack. Nature
Use-case posts that contrast 3D with 2D: Alpenglow Biosciences
The 3Di™ HOTLS delivers what many groups have wanted from light-sheet imaging, a practical way to combine whole-sample context with sub-cellular detail, while keeping the tissue intact and the workflow scalable. If your questions require complete morphology, true spatial relationships, and quantitative 3D readouts, a hybrid open-top approach is worth serious consideration.